
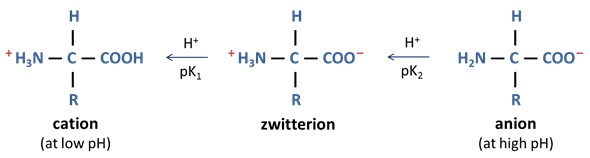
Since the charge on a weakly acidic group is always fractional with a negative sign (will be discussed in coming sections), its value always lie in the range of -1 to 0. NH 3 + is the conjugate acid of -NH 2 and is positively charged.Īt any pH, an acidic group can exist in either the uncharged, protonated (having an acidic proton, H +) form -COOH, or negatively charged, deprotonated (has lost its acidic H +) form -COO –, but never in the positively charged form. NH 2 is the basic group at N-terminal of the amino acids, or in R-group of the basic amino acids COO – is the conjugate base of -COOH, and negatively charged. Where -COOH is the acidic group at C-terminal of the amino acids, or in R-group of the acidic amino acids The generalized reaction of weakly acidic and basic groups of amino acids can be written as. It is advised to always use the pKa values mentioned in the question or your textbook itself but not using any outside reference value. The pKa values may differ among the reference sources, thus may lead to variation in the result for the same amino acid. pKa 1, pKa 2, and pKa 3 are the pKa value of the C-terminal -COOH group (a-carboxyl group), N-terminal -NH 2 group (a-ammonium group) and the R-group (if any) of the specified amino acid. The pKa table of amino acids lists a maximum of three pKa values, namely pKa 1, pKa 2, and pKa 3. An ionizable group may act as either a weak acid or a weak base depending on its ability to donate or accept a proton (H +), respectively.

The acidic, as well as the basic amino acids, have an additional ionizable group- their acidic or basic R-groups, respectively. a -NH 2 group (-NH or imine or secondary amine group in proline) at N-terminal, and II. In this context, the amino acids are categorized depending on the ionizability of their R-groups as follow-Īll the standard amino acids have at least two ionizable groups- I. Though their R-groups are around 10 4 to 10 6 times weaker acid than those of carboxylic acid groups of amino acids, they act as the catalytic residue at the catalytic center of some enzymes. These two amino acids have acidic side chains because they can donate a proton under suitable conditions. So, we will treat these two amino acids as acidic amino acids. At pH values close to or above their respective pKa, ignoring the charges on the side chain of these residues would lead to significant errors in charge and pI values. The side chain of tyrosine (pKa = 10.10) bears -0.44 and -0.99-unit charge at pH 10.0 and 12.0, respectively. For example, the side chain of cysteine (pKa = 8.14) bears -0.19 and -0.99-unit charge at pH 7.50 and 10.0, respectively. Though the side chains of tyrosine and cysteine are mostly uncharged at the neutral pH, they may bear non-zero charge at a pH near to or above their respective pKa values. The side chain of a basic amino acid generally acts as a weak base and may accept an H + from the solution. The side chain of an acidic amino acid generally acts as a weak acid and may donate an H + to the solution. The side chain of a neutral amino acid is unionizable at all pH.

They are also classified into neutral, acidic and basic amino acids depending on the acid-base behavior of their R-groups. IntroductionĪmino acids are classified into nonpolar (hydrophobic), polar-uncharged and polar-charged depending on the polarity and charge on their side chain at the neutral pH.
Calculating Exact Charge & pI of Amino acids, Peptides and other Molecules 1.


 0 kommentar(er)
0 kommentar(er)
